Chemical Division
The Company has set up its plant for the manufacture of Sodium Hydrosulphite (SHS), with technical know-how from the M/s Mitsubishi Gas chemical(Part of Mitsubishi group Japan), through the unique Sodium Formate route, adopted for the first time in India. The plant is located at Kovilur, Karaikudi in the State of Tamil Nadu, India. The nearest sea port is Tuticorin.
The product, Viz., Sodium Hydrosulphite, has a very wide application in industries like Textiles, Jaggery, Pharmaceuticals, Paper, Pulp and Ceramics.
The Company also set up a plant for the manufacture of Liquid Sulphur Dioxide, as a captive plant, with the technical know-how from M/s Garbato Implanti Chimici s.r.l., Italy.
SODIUM HYDROSULPHITE Material Safety Data Sheet (MSDS)
TCP Limited flagship product is Sodium Hydrosulphite which is produced through Sodium Formate process which is advance / latest technology. The conventional method of producing SHS is by Zinc (or) Amalgam process. The advantage of Sodium Formate process is that the end product does not contain even traces of heavy metals such as Zinc, Mercury, etc. Our technical partners MGC, Japan supplied both process know-how and equipments.
A. Identification of the substance:
Liquid Sulphur dioxide is one of the raw material used in manufacture of Sodium Hydrosulphite(SHS).
| Chemical Properties | Values |
|---|---|
| CAS Number | 7775-14-6 |
| Testing Method | IS : 1919 - 1982 |
| Molecular Formula | Na2S2O4 |
| Molecular Weight | 174.114 g/mol |
| Structural Formula | 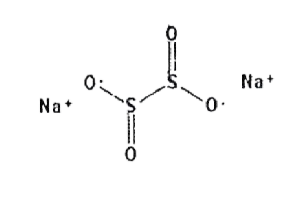 |
| Synonyms | Disodium hydrosulfite Dithionous acid Disodium salt Sodium dithionite Sodium hydrosulfite Sodium hyposulfite Sodium-sulfoxilate Hydroline |
B. Applications of Sodium Hydrosulphite:
Sodium Hydrosulphite is a reducing agent and the principle of its action is given by the following equation.
It can be used wherever the reducing action is required. It is used as reducing, bleaching and discoloring agent, in following industries.
- Textile: Sodium Hydrosulphite is extensively used as a reducing agent in VAT and Indigo dyeing. Its is used to penetrate the dye into fabrics and also used as a stripping agebt to remove uneven dyeing. In both cases the insoluble quinone form of Indigo is reduced to soluble Leuco form.
- Drugs & Pharmaceutical: In drugs & pharmaceuticals, SHS is used as a bleaching agent for reduction of nitro group into amino gropu and also used as a discoloring agent in the manufacture of antibiotic and analgesics drugs.
- Paper & Pulp: SHS is extensively used in Bleaching of paper and pulp.
- Sugar & Jaggery: For purification as a Bleaching Agent in the removal of organic and other impurities.
- Food: As a preservative.
-
Leather Industries:
In leather industries, Chromium is removed from the effluent by reducing soluble hexavalent chromium ion into insoluble trivalent chromium ion by the addition of Sodium Hydrosulphite.
2 Na2CrO4 + 3 Na2S2O4 + 4 H2O -----> 2 Cr(OH)3 + 4 Na2SO3 + 2 NaHSO3
-
Clay:
In clay, the dull colour is due to the presence of Iron. Addition of Sodium Hydrosulphite reduces the insoluble Ferric ion to soluble Ferrous ion and on subsequent washing iron is removed from clay.
Na2S2O4 + 2Fe3+ + 4H2O -----> 2NaHSO4 + 2Fe2+ + 6H+
C. Physical & Chemical Properties:
| Property | Value |
|---|---|
| Physical State | Solid Powder |
| Appearance | White |
| Odor | Faint sulphurous odor |
| Water solubility | 225 g/l at 20 oC |
| Melting Point | 300 oC / 572 oF |
| Decomposition Temp | 52 oC |
D. Packaging:
25kgs, 50kgs & 100kgs - Mild steel drums with polythene inner liner
25kgs, 50kgs, 10kg, 5kg, & 1kg - PVC packing with inner polythene liner
1kg - Pouch packing
50kgs, 25kgs & 100kgs - Carbuoy packing
LIQUID SULPHUR DIOXIDE Material Safety Data Sheet (MSDS)
Liquid Sulphur Dioxide is a colourless gas with a pungent odour. It is a liquid when under pressure, and it dissolves in water very easily. The material is supplied in liquified state in 900 kgs capacity cylinders. Although its chief uses are in the preparation of Sulphuric Acid, Sulphur Trioxide and Sulphites, Liquid Sulphur Dioxide is also used as disinfectant, refrigerant, reducing agent, bleach, and food preservative, especially in dry fruits.
A. Identification of the substance:
| Chemical Properties | Values |
|---|---|
| CAS Number | 7446-09-5 |
| Testing Method | IS-2432:1993 |
| Molecular Formula | SO2 |
| Molecular Weight | 64.066 g/mol |
| Compound Type | Acidic gas (Inorganic) |
| State | Liquid Acidic gas |
| Solubility | Soluble in water |
| Odour | Pungent, Sulphurous |
| Synonyms | Sulphurous acid Anhydride |
B. Application of Liquid Sulphur Dioxide:
Liquid Sulphur Dioxide is used in the following industries,
- Chemicals: In the production of Sodium Hydrosulphite,Sodim Formaldehyde, Zinc Formaldehyde, Sodium sulphoxilate, Sulphites, Sodium Bisulphites, Sodium Thiosulphates, Sulphonates, Chlorine Sulphonate Paraffins and Sulphuryl-Chloride, Sulphitated Aethanolamines, Chromium Basic Sulphate, Manganese Sulphate, Caprolactam, Resins, Dye Intermediates. Phtalic Anhydride (co-catalyst) and Acrylic Fibres (activator).
- Food: In the preservation and bleaching treatment of dried and candied fruit, fruit syrup, citrus fruit, cherries, apples, fruit juices, potatoes, cereals, mushrooms, animal fats and oils. In sugar industry as bacteriostatic, bleaching and acidifying agent. In starch production as bacteriostatic.
- Drinking Water Treatment: For removal the excess of chlorine.
- Waste Water Treatment: As reductant of polluting metallic ions (chrome) and as oxidant of Sulphide and Cyanides.
- Mining Industry: For extraction and refining (as floating agent) some metals from their respective ores.
- Metallurgical Industry: In order to produce a reducing environment during magnesium casting or as catalyst of resins in the manufacturing of casting moulds.
- Textile and Paper & Pulp Industry: As bleaching agent and for removing the excess of Chlorine and Peroxide.
- Pharmaceutical Industry: As antiseptic and disinfectant.
- Petroleum Refinery: To remove (as solvent) sulphur, oxygen and nitrogen containing compounds from kerosene and light oils.
- Electric Power Stations: For conditioning stack gas to facilitate removal of fly ash.
- Glass Manufacture: As an acidifying agent.
C. Packaging:
900kgs - Net in returnable tonners [Cylinders]
SODIUM THIOSULPHATE Material Safety Data Sheet (MSDS)
Sodium Thiosulphate also called as Thiosulfuric Acid or Disodium Salt is an inorganic salt which is also available in the Pentahydrates. This chemical substance has a chemical formula of Na2S2O3.5H2O . It appears as a bright white colourless crystal or even in powder form. The substance is known to possess Alkaline nature when decomposed to sulphide and sulphate in the air.
A. Identification of the substance:
| Chemical Properties | Values |
|---|---|
| CAS Number | 7772-98-7 |
| Testing Method | IS : 14781 |
| Molecular Formula | Na2S2O3.5H2O |
| Molecular Weight | 248.11 g/mol |
| Compound Type | White Crystalline Powder (Inorganic) |
| State | Solid |
| Solubility | Soluble in water |
| Synonyms | Sodium Hyposulphite , Disodium Thiosulphate. |
B. Applications of Sodium Thiosulphate:
- Photography: This chemical substance is often used as a fixing agent in photography. It helps in dissolving the silver salts from the negatives.
- Cleansing Agent: When dissolved in an enormous quantity of warm water, this chemical substance can be used as a cleansing agent.
- Industries: Sodium Thiosulphate is popularly used for the de-chlorination of small water bodies such as aquariums, ponds, and so on. This inorganic substance is highly used in the production of patinas. It is widely used in gold extraction from its ores.
- Medical Field: It is used in pharmaceutical preparations, including anionic surfactant helping in dispersion. Additionally, it is a crucial antidote to cyanide poisoning. It aids in treating ringworm plus overcoming the side-effects of chemotherapy.
Apart from the above applications, Sodium Thiosulphate structure shows that the compound is preferably used in water treatment, neutralizing bleach, leather tanning, photographic film processing, and chemical heating pads, and so on.
C. Packaging:
50kgs - HDPE Woven bags with heat sealed inner liner
SODIUM FORMATE Material Safety Data Sheet (MSDS)
The Sodium Formate we are supplying is a byproduct in production of Sodium Hydrosulphite. It is an organic sodium salt which is the monosodium salt of formic acid . It has a role as a buffer and an astringent. Sodium Formate is used in fabric dyeing and printing processes. It is also used as a buffering agent for strong mineral acids to increase their pH, as a food additive(E237), and as a de-icing agent. It cleans and softens leather and creates superior finishes, such as suede. Sodium Formate is also used in several steps of leather tanning, where it acts as a general pH-neutralizer.
A. Identification of the substance:
| Chemical Properties | Values |
|---|---|
| CAS Number | 141-53-7 |
| Testing Method | IS : 13475(1992) |
| Molecular Formula | HCOONa |
| Molecular Weight | 68.01 g/mol |
| Compound Type | White Crystalline Powder (Organic) |
| State | Solid |
| Solubility | Soluble in water |
B. Application of Sodium Formate:
- A tanning agent in leather industry .
- Oil Drilling field / Oil field chemicals
- Pulp and paper industry
- In production of Sodium Hydrosulphite
- For fabric dyeing and printing in textile industry
- In manufacturing of formic acid
C. Packaging:
50kgs - HDPE Woven bags with heat sealed inner liner
SODIUM SULPHITE Material Safety Data Sheet (MSDS)
Sodium Sulphite is a white powder or crystalline solid with no odour but a slightly salty taste. The compound is stable in dry air, but tends to decompose in moist air to produce Sulphur Dioxide (SO2) and Sodium Hydroxide (NaOH). The compounds are used in photography, bleaching of wool, as a preservatives in foods, beverages, and medications. It act as effective antioxidant compounds and is also used in the manufacture of pulp for paper and wood products.
A. Identification of the substance:
| Chemical Properties | Values |
|---|---|
| CAS Number | 7757-82-6 |
| Testing Method | IS : 247(1987) |
| Molecular Formula | Na2SO3 |
| Molecular Weight | 126.04 g/mol |
| Compound Type | Salt (Inorganic) |
| State | Solid |
| Solubility | Soluble in water, Slightly in ethyl alcohol. |
| Synonyms | Disodium sulphite |
B. Applications of Sodium Sulphite:
- As an Oxygen scavenger agent
- Used to avoid corrosion problems by treating water that is fed to steam boilers.
- In the textile industry, it is used as a reducing agent for bleaching.
- Desulfurizing and dechlorinating swimming pools.
- It is used as a bleaching agent in the paper and pulp industry. It helps break down lignin and other impurities, giving a brighter and whiter paper.
- It also acts as an oxygen scavenger in paper production. Oxygen can cause the oxidation of cellulose and other components of paper which causes discoloration. Sodium Sulphite prevents this from happening.
- It can be used as de-chlorinating agent in water treatment.
- It is utilized in the textile industry as a bleach, de-chlorinator or de-sulphurizer and in the tanning of leather.
C. Packaging:
50kgs - HDPE Woven bags with heat sealed inner liner